Metallic Bonding
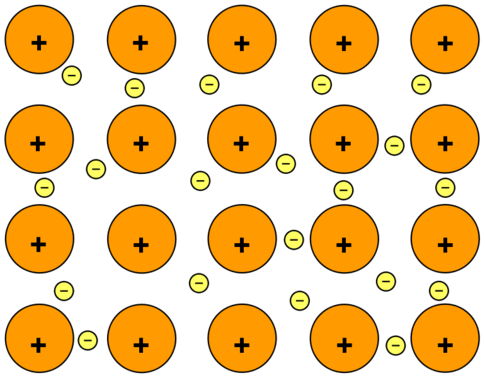
Metallic Bonding
Force of attraction, which holds positively charged metal ions surrounded by a sea of free moving electrons, is called a metallic bond. Metallic bonding depends upon the sea of free moving electrons.
Physical Properties Of Metals:
They can conduct electricity efficiently in solid or molten states, as they have a sea of electrons moving around randomly around the positively charged metal. It can even conduct electric current from harmful to the positive terminal. On the application of sufficient force, layers of atoms can slide over one another in the metallic compound, which implies that they are potent substances and are flexible enough to form any shape or made into wire devoid of breakage, giving them two properties particular to these compounds malleability and ductility respectively. They have high melting and boiling points and are hard. They are insoluble in water and organic solvents.
